The ozone layer, a critical component of Earth’s atmosphere, was first identified in 1913 by French physicists Charles Fabry and Henri Buisson. They noticed a significant amount of ultraviolet (UV) radiation missing from the solar spectrum, which led to the discovery of ozone (O3) as the responsible agent. This discovery was further advanced by British physicist Gordon M. B. Dobson, who developed specialized spectroscopy equipment to measure atmospheric ozone from the ground and established a global monitoring network still active today, with ozone levels measured in “Dobson units.”
Ozone forms high in the atmosphere when UV radiation splits oxygen molecules (O2), allowing individual oxygen atoms (O) to combine with O2 and form O3. Even in small concentrations (about ten parts per million), ozone plays a vital role in shielding life on Earth from the Sun’s harmful UV rays. Without it, the UV light would rapidly break down essential organic molecules.
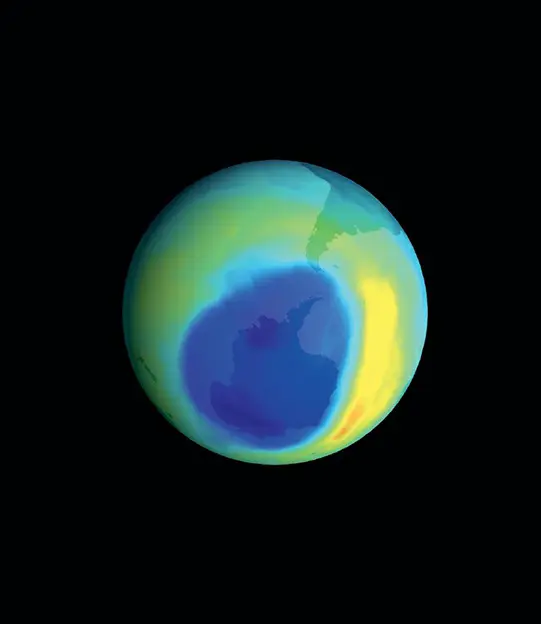
In the 1970s, scientists discovered that chlorofluorocarbons (CFCs), human-made chemicals used predominantly as refrigerants, were depleting the ozone layer, particularly over the Earth’s poles. This led to the formation of “ozone holes.” The international community responded with environmental stewardship and corporate responsibility, phasing out CFCs in favor of safer alternatives. As a result, the ozone layer has been gradually recovering, showcasing a rare but significant triumph in environmental conservation.